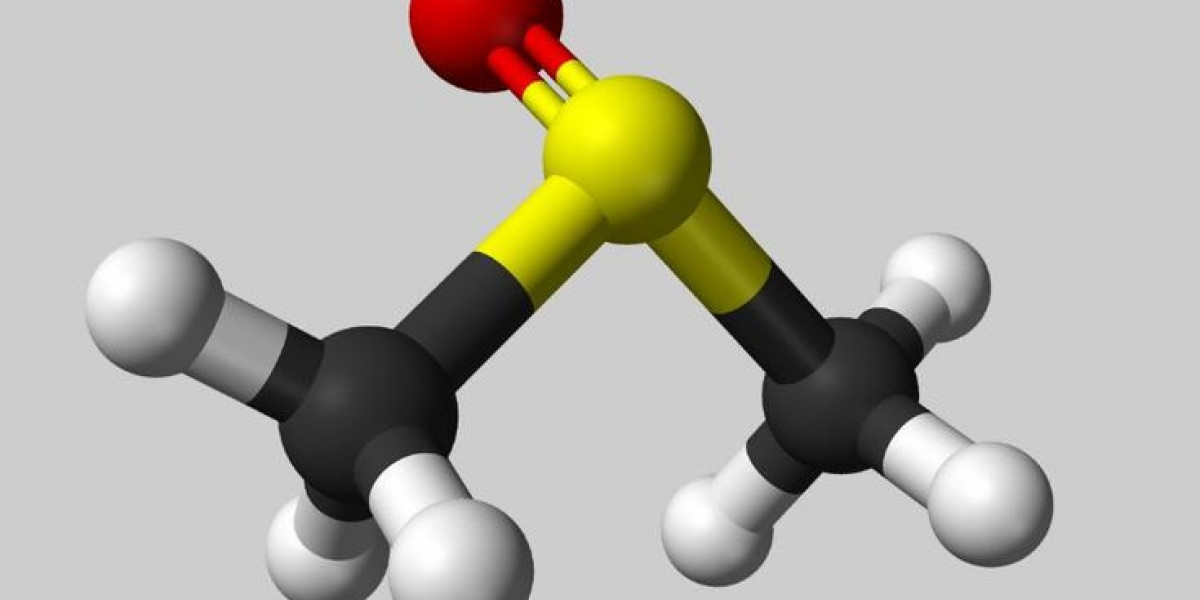
Diethyl Sulfide is an organic compound with the formula (C2H5)2S. It is a colorless liquid with an unpleasant odor resembling that of rotten eggs or cabbage.
Physical and Chemical Properties
Diethyl Sulfide is a volatile, flammable liquid at room temperature. Its melting point is -115°C and boiling point is 116°C. It is slightly soluble in water but highly soluble in organic solvents like alcohol and ether. Chemically, it is stable under normal conditions but can undergo oxidation reactions when exposed to air for prolonged periods. It readily burns in air or oxygen with a blue flame to give carbon dioxide, water and sulfur dioxide as products. It has a density of 0.849 g/cm3 and refractive index of 1.437 at 20°C.
Production
Commercially, it can be produced through the reaction of sodium sulfide (Na2S) with Diethyl Sulfide ((C2H5)2SO4) in ethanol solvent. This reaction proceeds at a moderately high temperature of around 80°C:
Na2S + (C2H5)2SO4 → (C2H5)2S + Na2SO4
The sodium sulfate produced acts as a separating agent and it separates as the upper layer which is then purified by distillation. Alternatively, it can also be synthesized through the reaction of hydrogen sulfide (H2S) gas with diethyl chloride (C2H5Cl) in the presence of a strong base like sodium hydroxide.
Applications
Some key applications of it include:
- Flavor and Fragrances: Due to its pungent aroma, Diethyl Sulfide finds extensive use in the flavor and fragrance industry. It imparts flavors reminiscent of cabbage and garlic.
- Solvents: It serves as an excellent solvent for fats, waxes, gums and resins. It is commonly used as a solvent for dissolving and applying fragrances in detergents, cosmetics and household products.
- Chemical Intermediate: It acts as an important starting material in the production of several sulfur-containing chemicals, pharmaceuticals, pesticides and dyes.
- Extractions: The good solvent properties of it allow it to be used for extracting essential oils from plants and for separating various organic compounds.
Safety
Being volatile and flammable in nature, it must be handled with care. The exposure limits set for Diethyl Sulfide are 10 ppm (8hr time-weighted average) and 15 ppm (15 minute short term exposure limit). Prolonged and repeated exposure to its vapors can cause nausea, headaches, dizziness and irritation to eyes, nose and throat. It is recommended to use Diethyl Sulfide in well-ventilated areas and away from possible ignition sources like open flames. Skin contact must be avoided as it can cause defatting of skin upon prolonged contact. In case of accidental exposure, affected areas should be rinsed with plenty of water. Proper protective gear like goggles, gloves and mask should be worn while handling Diethyl Sulfide.
Get More Insights Diethyl Sulfide
For Better Understanding, choose preferred language-
About Author-
Money Singh is a seasoned content writer with over four years of experience in the market research sector. Known for her strong SEO background, she skillfully blends SEO strategies with insightful content. Her expertise spans various industries, including food and beverages, biotechnology, chemical and materials, defense and aerospace, consumer goods, etc. (https://www.linkedin.com/in/money-singh-590844163)