Safeguarding Patient Health: Unpacking the Mycoplasma Testing in Clinical Market
Global Mycoplasma Testing in Clinical Market, By Products (Kits and Reagents, Instruments, Services), Technique (Microbial Culture Techniques/Direct Assay, Polymerase Chain Reaction, ELISA, DNA Staining/Indirect Assay, Enzymatic Methods), Application (Cell Line Testing, Virus Testing), Disease Area (Respiratory, Urogenital, Gastrointestinal, Musculoskeletal, Cardiovascular, Others), End User (Diagnostic Laboratories, Hospitals) – Industry Trends and Forecast to 2030.
Introduction: Mycoplasmas are small, cell wall-less bacteria belonging to the class Mollicutes. While some species are commensals, others are significant human pathogens capable of causing a range of respiratory, urogenital, and systemic infections. Accurate and timely detection of mycoplasma infections is crucial for effective clinical management, preventing disease progression, and controlling transmission. The mycoplasma testing in the clinical market encompasses the various diagnostic assays and kits used to identify these elusive microorganisms in clinical specimens.
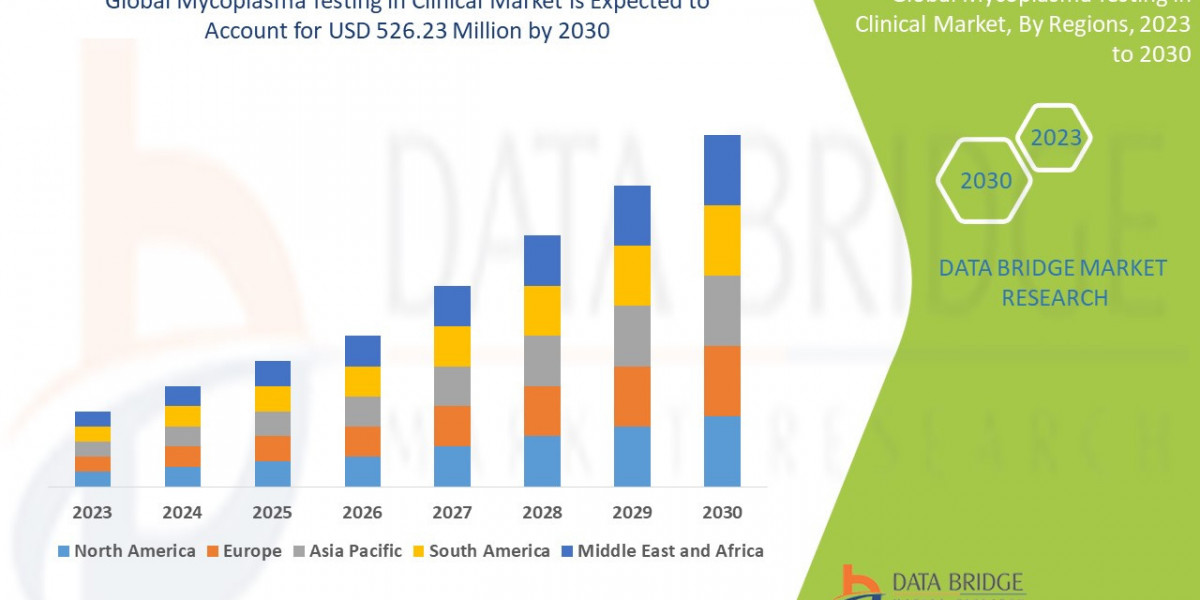
Market Size: Data Bridge Market Research analyses that the mycoplasma testing in clinical market which was USD 295.06 million in 2022, is expected to reach USD 526.23 million by 2030, at a CAGR of 7.5% during the forecast period 2023 to 2030. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
Market Share: Analyzing the market share reveals a competitive landscape with a mix of established diagnostic companies and specialized microbiology assay providers. The market share is influenced by factors such as the type of testing technology offered (nucleic acid amplification tests (NAATs), serological assays, culture-based methods), the sensitivity and specificity of the assays, the ease of use, the availability of automated platforms, and the geographical presence of the manufacturers. Nucleic acid amplification tests (NAATs), particularly PCR-based assays, currently hold a significant share due to their high sensitivity and rapid turnaround time. However, other methods like culture and serology still maintain a presence in specific clinical settings. The increasing demand for point-of-care testing solutions is also influencing market share dynamics.
Market Trends: Several key market trends are shaping the evolution of the mycoplasma testing in the clinical market. A prominent trend is the increasing adoption of nucleic acid amplification tests (NAATs) due to their superior sensitivity, specificity, and speed compared to traditional culture-based methods. Multiplex PCR assays, which allow for the simultaneous detection of multiple mycoplasma species, are also gaining popularity. Another significant trend is the development and commercialization of automated or semi-automated platforms for mycoplasma testing, improving workflow efficiency and reducing the risk of human error in clinical laboratories. The growing awareness of the role of Mycoplasma genitalium as a sexually transmitted pathogen is driving increased testing demand in sexual health clinics. Furthermore, the development of more rapid and user-friendly point-of-care testing (POCT) solutions for mycoplasma detection is an emerging trend with the potential to decentralize testing and facilitate quicker diagnosis and treatment initiation. The integration of molecular diagnostics with digital platforms for data management and analysis is also a developing trend in this market.
Market Growth: The mycoplasma testing in the clinical market is experiencing consistent market growth, driven by the increasing recognition of the clinical importance of mycoplasma infections across various medical specialties. The advancements in diagnostic technologies, particularly the widespread adoption of NAATs, are significantly contributing to this growth by offering more accurate and timely detection. The rising prevalence of certain mycoplasma-related infections and the growing emphasis on accurate diagnosis for effective patient management are also fueling market growth.
Market Demand: Market demand for mycoplasma testing in clinical settings is primarily driven by the need for accurate diagnosis of respiratory infections (e.g., Mycoplasma pneumoniae), urogenital infections (e.g., Mycoplasma genitalium, Ureaplasma urealyticum), and systemic infections in immunocompromised individuals. The demand is also increasing due to the recognition of mycoplasma's role in certain chronic conditions and the need for differential diagnosis in patients presenting with relevant symptoms. The growing awareness among clinicians about the availability and benefits of advanced mycoplasma testing methods, particularly NAATs, is further driving demand.
Factors Driving Growth: Several fundamental factors driving growth of the mycoplasma testing in the clinical market. Firstly, the increasing awareness among healthcare professionals about the clinical significance of mycoplasma infections, including their role in respiratory illnesses, urogenital infections, and complications in immunocompromised patients, is a major driver. Secondly, the superior performance characteristics of nucleic acid amplification tests (NAATs), such as high sensitivity, specificity, and rapid turnaround time, are leading to their increased adoption and driving market growth. Thirdly, the rising prevalence of certain mycoplasma-related infections, such as Mycoplasma genitalium infections, is directly increasing the demand for diagnostic testing. Fourthly, the development and availability of automated and semi-automated testing platforms are improving the efficiency and scalability of mycoplasma testing in clinical laboratories, further contributing to market growth. Finally, the growing emphasis on accurate and timely diagnosis for effective patient management and infection control is a key factor driving the demand for reliable mycoplasma testing solutions. The increasing focus on sexual health and the recognition of Mycoplasma genitalium as a significant STI are also contributing to market expansion.
In conclusion, the mycoplasma testing in the clinical market is a vital and growing sector within infectious disease diagnostics. Driven by increasing awareness of mycoplasma's clinical impact, advancements in molecular diagnostic technologies, and the need for accurate and timely detection, the demand for reliable mycoplasma testing solutions is poised for continued growth, ultimately contributing to improved patient care and public health.
Browse Trending Reports:
Intelligent Building Automation Technologies (IBAT) Market
Sulfa Drugs Market
Trichloroacetone Market
Polyvinyl Chloride (PVC) Dunnage Trays Market
Digital Mobile X-ray Devices Market
Chronic Obstructive Pulmonary Disease (COPD) Drug Market
Benzodiazepine Drugs Market
Farm Equipment Rental Market
Egg Yolk Lecithin Market
Automotive Windshield Washer System Market
Face Shield Market
Supplements and Nutrition Packaging Market
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC : +653 1251 975